Golden Bridge helps medical enterprises planning and preparing to enter the Chinese market to complete the entire registration process quickly and efficiently, and successfully obtain the product registration certificate.
Golden Bridge has 22 years of experience in regulatory registration, professional regulatory team, under the complex regulatory system, Golden Bridge can provide you with the whole registration process support, help you complete the registration goal on time, successfully enter the market.
Our services include:
- Product regulation research and evaluation
- Develop a product registration plan
- Registration test
- Clinical trials
- Registration information and application process assistance
- Project full cycle registration management
- Review regulation ideas and criteria
- Professional legal advice
- Efficient communication and timely response

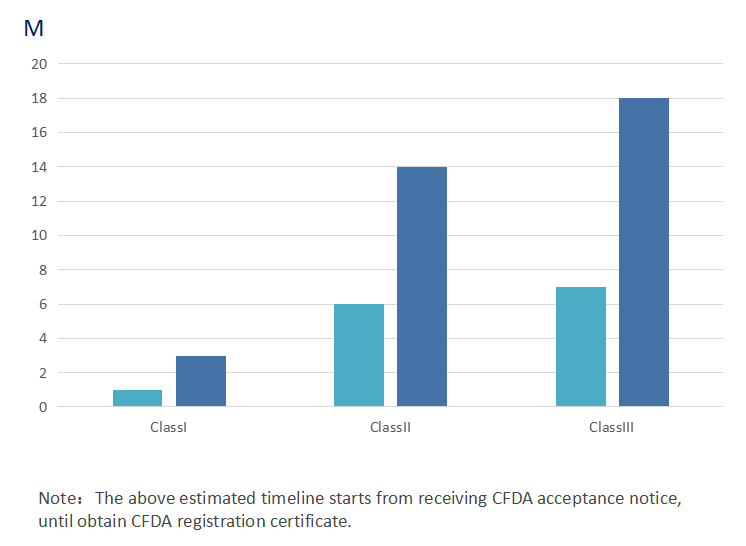
Regulatory Approval Process

NMPA Registration Timeline